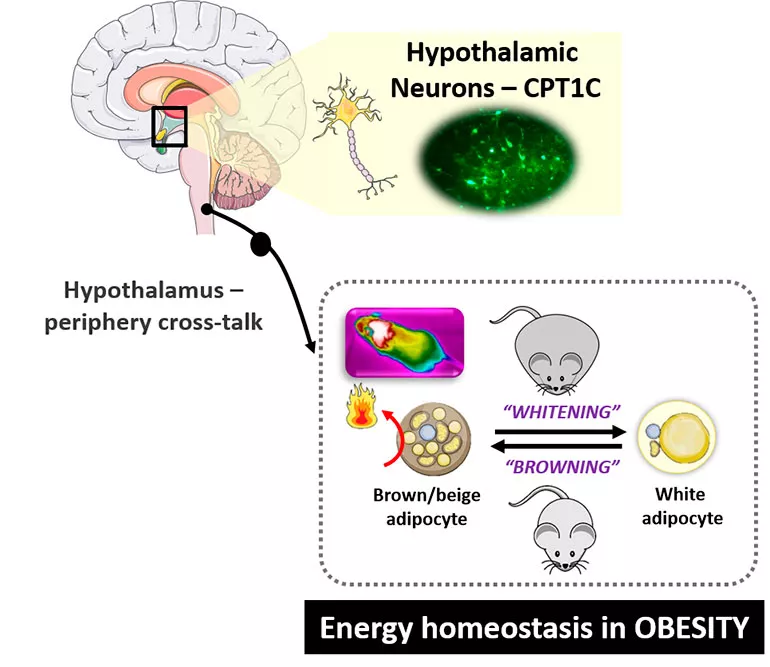
The hypothalamus is the main regulator of energy homeostasis in response to nutrients, to control energy use and expenditure. Our group is interested in evaluating how neurons in the hypothalamus, especially in the mediobasal hypothalamus (MBH), respond to metabolic challenges such as an overload of fatty acids, a high fat diet or fasting to further regulate peripheral metabolism. We are investigating the molecular mechanisms by which hypothalamic neurons respond to changes in lipids (i.e. ceramides, endocannabinoids, fatty acids) and neuropeptides (i.e. leptin and ghrelin) in the brain and how these hypothalamic signals regulate food intake, overall energy expenditure, brown adipose tissue thermogenesis as well as fatty acid metabolism in the liver, muscle or white adipose tissue.
This hypothalamic dynamic is especially relevant in the development of chronic metabolic diseases, including obesity and type 2 diabetes, which are highly prevalent today. We are therefore trying to identify new targets in the hypothalamus to fight against these metabolic disorders. Specifically, one of our group’s main lines of research focuses on the neuronal isoform carnitine palmitoyltransferase 1 C (CPT1C). This protein is expressed in neurons and, despite the lack of catalytic activity, it plays a critical role in the obesogenic phenotype. Our group has demonstrated that CPT1C KO mice show an impaired ceramide metabolism in neurons, a key step in the signalling pathway of ghrelin and leptin in the hypothalamus. Moreover, CPT1C in the ventral hypothalamus is necessary for the proper sensing of a negative energy balance and fuel partitioning in liver and muscle. Our aim is now to elucidate the neuronal function of CPT1C in the hypothalamus in responding to metabolic adaptations and to prevent an obesogenic phenotype. In addition, we are looking for nanomedicine-based approaches that are capable of modifying CPT1 in the hypothalamus.
The Neurolipid Lab uses biochemical and molecular techniques to understand the molecular mechanisms by which the hypothalamus senses and responds to metabolic challenges. Specifically, we are using obese mice models to develop in vivo determinations (e.g. BAT thermogenesis, central administration of drugs or hormones, hypothalamic ceramides and endocannabinoids metabolism, among others).